
DIGITAL DUET & TRIO INFO KIT
Lightening the path to health
Brochures, Clinical Studies, Press Releases, and more…

Download your Info Kit Now!
SEE THE FUTURE OF LASER THERAPY 
![]()
Capture digital images and videos (including crisp voice recording) before, during, and after the treatment. With CPT codes for imaging, practices are also given an additional revenue stream opportunity.
![]()
Streamline workflow and optimize practice efficiency through the automated export of images, videos, and reports directly to your EMR system*. Also, email export support is available for images and reports.
![]()
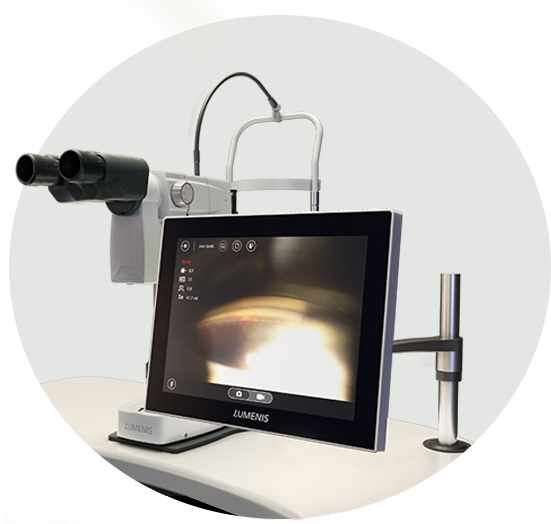
Enable technological leadership with the first and only digital SLT+YAG device, featuring an HD camera, touch screen, and a best-in-class “smart-phone” user interface.
![]()
Provide safe and efficient treatment through advanced optics and super-gaussian beam profile (a lower optical breakdown).
![]()
Communicate with Lumenis’ expert engineers through remote support, to ensure minimal downtime and optimal focus on your clinical practice.
![]()
Generate automated, detailed, and personalized treatment reports that allow you to minimize staff and administrative time.
* Connectivity is through a repository folder with acompatible format between the Digital Duet/Trio device and most EMR systems.

Digital Trio includes a retinal care photocoagulator, Smart532, with SmartPulse sub-threshold technology, ensuring efficacy, and minimizing collateral damage. This all-in-one solution allows physicians to expand retina treatments as well as to further optimize iridotomy.
Indication for Use:
In EU: Evaporative Dry Eye Disease (DED), also known as dry eye syndrome or lipid tear deficiency, due to Meibomian Gland Dysfunction (MGD). This indication is intended for Fitzpatrick skin types I-V.
In US: Improvement of signs of Dry Eye Disease (DED) due to Meibomian Gland Dysfunction (MGD), also known as evaporative dry eye or lipid deficiency dry eye, in patients 22 years of age and older with moderate to severe signs and symptoms of DED due to MGD and with Fitzpatrick skin types I-IV. IPL is to be applied only to skin on the malar region of the face, from tragus to tragus including the nose (eyes should be fully covered by protective eyewear). IPL is intended to be applied as an adjunct to other modalities, such as meibomian gland expression, artificial tear lubricants and warm compresses.
The indications are only relevant where they were approved by the Regulatory Authorities.
Treatment with OptiLight is contraindicated for patients with the following conditions in the treatment area: Ocular surgery or eyelid surgery or Neuro-paralysis within 6 months prior to the first treatment; Uncontrolled eye disorders affecting the ocular surface; Pre-cancerous lesions, skin cancer or pigmented lesions; Uncontrolled infections or uncontrolled immunosuppressive diseases; Recent Ocular infections; History of cold sores or rashes in the perioral area, including: Herpes simplex 1 & 2, Systemic Lupus erythematosus and porphyria; Use of photosensitive medication and/or herbs that may cause sensitivity within 3 months prior to the first IPL session; Recent radiation therapy to the head or neck or planned radiation therapy; Recent treatment with chemotherapeutic agent or planned chemotherapy; History of migraines, seizures or epilepsy. Patients eyes must be completely occluded during the treatment. Please refer to the operator manual for a complete list of intended use, contraindications and risks.
The following possible side effects can occur following IPL treatments: Pain/discomfort, damage to natural skin texture, change of pigmentation, scarring, excessive edema, fragile skin, bruising, burns, pruritus and xerosis. Please refer to the user manual or ask your doctor for a complete list of intended use, contraindications and risks.
Copyright © 2023 Lumenis Be Inc. All Rights Reserved